"Unable to make drugs" targets finally become history, KRAS inhibitor breakout contest kicks off
September 17, 2021
Recently, Cinda Biotech and Jinfang Pharmaceutical reached a licensing agreement to obtain the latter's exclusive global development and commercialization rights for the KRAS G12C inhibitor GFH925 in China (including Mainland China, Hong Kong, Macau and Taiwan), and has global development and commercialization The right to choose rights. KRAS was once considered a "non-drugable target", but Amgen's AMG510 was approved for marketing in May 2021, breaking the curse of "non-drugging" and providing a new drug option for patients with KRAS mutations. There are also a number of domestic pharmaceutical companies that have deployed KRAS targets, including Betta Pharmaceuticals, Yifang Biological, Jiaxian, Jinfang Pharmaceutical, etc.
Overview of R&D progress of some KRAS around the world
Data source: Yaozhi data, corporate announcements and other public information
(1) Overview of KRAS targets
RAS (rat sarcoma) is a low-molecular-weight (21kDa) guanine nucleotide binding protein with GTPase activity located on the cell membrane, including three subtypes of HRAS, NRAS and KRAS. In the human body, RAS is like a gene "switch". By combining with guanine trinucleotide phosphate (GTP) or guanine dinucleotide phosphate (GDP), it can be adjusted between activation and inactivation. Cell growth, proliferation, differentiation, senescence, apoptosis, etc. In healthy cells, the cycle of RAS binding to GDP/GTP is very slow. When receiving a signal, RAS will bind to the recruited guanine nucleotide exchange factor on the cell membrane, release GDP, and quickly bind to GTP to enter the "on" state. Afterwards, under the action of the GTPase activating protein, the activity of GTPase is greatly enhanced, and the GTP bound to RAS is hydrolyzed into GDP and enters the "off" state again. In tumor cells, due to the loss of the intrinsic GTPase (GTPase) activity, GTP cannot be hydrolyzed into GDP. Therefore, RAS is always in the "on" state, continuously activating downstream pathways, causing cancer cells to have problems such as malignant proliferation and anti-apoptosis.

Figure: RAS switch icon
Data source "Targeting the untargetable KRAS in cancer therapy"
For the RAS mutation, the hydrolysis of GTP is the key to the binary switch. However, in research and development, scientists found that RAS is difficult to make medicines. On the one hand, the affinity constant of RAS and GDP/GTP can reach picomolar level, but the concentration of GTP in cells is only millimolar, which makes it difficult for small molecule drugs to compete with the substrate. Binding; On the other hand, the surface of the RAS protein lacks a cavity that facilitates the binding of small molecules. In view of this, scientists set their sights on the KRAS gene. The frequency of KRAS mutations is significantly higher than that of NRAS and HRAS mutations, and the mutation rate accounts for 85% of RAS mutations. Point mutations are the most common way of mutations in KRAS genes, especially codon 12 mutations are the most common, including G12C, G12D, etc., of which KRAS G12C accounts for 44% of KRAS mutations. When the KRAS gene is mutated, the conformation of the KRAS protein encoded and expressed will change, causing the KRAS protein to almost completely lose its intrinsic GTPase enzyme activity, the dissociation of GTP from KRAS is reduced, and the binding capacity of GDP and KRAS is weakened, so that the KRAS protein is always activated. State, continue to activate downstream pathways, and promote the occurrence and development of tumors.
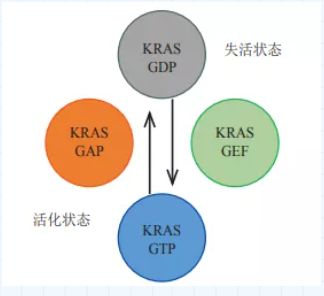
Figure: KRAS activation and inactivation
Data source "The specific function of Kras in cancer initiation"
Aiming at the bottleneck of the lack of binding pockets on the KRAS protein surface, the "tethering" technology finally broke the ground. The basic principle of "tethering" is to screen targets containing cysteine by using disulfide bond-containing small molecule fragment libraries-after the disulfide bond of the small molecule fragment is exchanged with the Cys sulfhydryl group on the KRAS protein The small molecule fragments are connected to the KRAS protein using Cys as a "bridge". The small molecule fragments find a suitable binding cavity to attach to the protein, and finally remove the cysteine through a reduction reaction. With this method, a small expandable pocket was finally found in the cysteine and molecular switch II region (S-IIP) near the 12th codon mutation of the KRAS protein. This pocket became a breakthrough point for later drug design.
(2) Global layout of KRAS G12C inhibitors
(1) AMG510 is the first oral inhibitor targeting KRAS G12C approved for marketing. It targets the hidden grooves on the surface of the KRAS protein, and can irreversibly bind to the KRAS mutant protein Cys 12, and is firmly locked so that GDP cannot be activated to become GTP. Thereby preventing the proliferation of tumor cells.

Picture: AMG510
Data source Amgen
In May 2021, Amgen's AMG510 (Sotorasib) was approved by the FDA for marketing, breaking the curse of KRAS "not druggable". The indication is the second-line treatment of non-small cell lung cancer with KRAS G12C mutation. The approval of AMG510 is based on the CodeBreak 100 Phase II clinical study. The trial included 124 patients with advanced NSCLC with KRAS G12C mutations. The trial ORR reached 37.1%, CR reached 2.4%, PR reached 34.7%, DCR reached 80.6%, and DoR was 10 months. . In terms of safety, the main adverse events of AMG510 were all grade 1 mild, and only 2 cases of grade 3 treatment-related adverse events were anemia and diarrhea. On ASCO 2020, Amgen announced the Phase I clinical data of AMG510 for the treatment of colorectal cancer (CRC). As of January 8, 2020, the trial included 42 patients with colorectal cancer who had previously received an average of 3 lines of treatment. The results showed that, The overall ORR was 7.1% (3/42), and the DCR was 76.2%. At a dose of 960 mg, ORR reached 12% (3/25) and DCR reached 80%. In terms of adverse reactions, adverse events above grade 3 include diarrhea (n=1) and anemia (n=1). In general, for CRC patients with KRAS G12C mutations who have previously received several lines of therapy, AMG510 monotherapy patients are well tolerated. (2) MRTX-849 (Adagrasib) is designed by Mirati Therapeutics, and its structure is similar to Amgen's AM510. In the KRAS protein molecule, the cysteine adjacent to the 12th codon mutation and the molecular switch II region (S-IIP) have a small expandable pocket. MRTX-849 irreversibly binds to Cys 12 through a covalent form, which will The KRAS G12C protein locks in the "off" state, thereby blocking KRAS signaling. On June 25, the US FDA granted MRTX-849 breakthrough therapy designation for the treatment of NSCLC patients with KRAS G12C mutations. MRTX-849 is expected to submit a new drug application to the FDA in the second half of 2021.

Figure: MRTX-849 chemical structure
Data source: Mirati Therapeutics
In the phase I/II trial code-named Krystal-1, MRTX-849 showed good anti-tumor activity and tolerable safety in a variety of tumors with KRAS G12C mutations. Among 18 CRC patients, the objective response rate of MRTX-849 was 17% (3/18), and the DCR was 94%. The data of MRTX-849 is very eye-catching, and it has the potential to surpass AMG510 to become BIC. In addition to monotherapy, MRTX-849 is exploring a combination program with drugs such as pembrolizumab or cetuximab. In addition, the development and commercialization rights of MRTX-849 in Greater China belong to Zai Lab. The advance payment for this transaction amounts to US$65 million, and the follow-up milestone expenses amount to US$273 million.
image.png
Figure: MRTX-849 mechanism of action
Data source: Mirati Therapeutics
(3) Eli Lilly entered the research and development of KRAS G12C inhibitors earlier, but the first KRAS G12C inhibitor LY3499446 was suspended in phase I clinical trials in 2020 due to toxicity issues. In 2021, Eli Lilly will make a strong comeback and announce LY3537982 with "high selectivity and potency" on AACR. Preclinical data shows that compared with AMG510 and MRTX849, LY3537982 has lower IC50 in KRAS G12C mutant H358 lung cancer cells, and its inhibitory activity is expected to be further improved. However, whether preclinical data can be successfully transformed in clinical trials remains to be clinically verified. LY3537982 plans to launch FIH in 2021.
(3) Domestic KRAS G12C inhibitor layout
According to statistics, so far, a total of 10 KRAS inhibitors have been declared clinically in China, of which 3 are imported and 7 are domestic. Currently, Novartis' JDQ443 and Jinfang Pharmaceutical GFH925 have been advanced to Phase II clinical trials, and the remaining products are still in IND or Phase I clinical phases. As the global BIG Pharma has successively deployed KRAS G12C inhibitors, the domestic research and development enthusiasm for KRAS G12C inhibitors has also been completely ignited. Betta Pharmaceuticals, Yifang Biological, Jiaxian, Jinfang Pharmaceutical, etc. have successively deployed.
BPI-421286 from Betta Pharmaceuticals is a new type of potent and highly selective covalent irreversible KRAS G12C oral small molecule inhibitor. Preclinical data shows that BPI-421286 can effectively inhibit the proliferation of tumors carrying KRAS G12C mutations, and exhibits good anti-tumor effects on a variety of transplanted tumor models carrying KRAS K12C mutations. In April 2021, BPI-421286 was approved for clinical use. Yifang Bio's D-1553 is the first KRAS G12C inhibitor independently developed and entered clinical trials in China. It has launched international multi-center phase I/II clinical trials in the United States, Australia, China and other countries, and will start domestically in April 2021. Approved clinically. Pre-clinical trials have shown that, compared with similar drugs under investigation, D-1553 has higher bioavailability and lower plasma protein binding rate. Jacos is currently developing three KRAS inhibitor projects, namely JAB-21822, JAB-22000 and JAB-23000, for the inhibition of KRAS with G12C, G12D and G12V mutations. In pre-clinical trials, JAB-21822 showed superior PK characteristics than AMG 510 and MRTX-849 and affinity for KRAS G12C, and has good therapeutic potential. On August 3, 2021, the clinical trial of JAB-21822 completed the first patient administration in China. In addition, the company is also exploring the therapeutic effects of the combination of KRAS inhibitors and SHP2 inhibitors.
(4) Summary
In view of the structural characteristics of KRAS mutants, the complexity of signal pathways, and the drug resistance of KRAS mutant tumors, KRAS was once called a "non-drug target". Amgen’s AMG-510 breaks the curse that KRAS mutations are incurable and provides a brand new medication option for patients with KRAS mutations. There are also a number of domestic pharmaceutical companies that are deeply involved in the KRAS G12C field. Yifang Bio and Betta Pharmaceuticals are currently in the leading R&D progress and are both in clinical phase I, and more than ten companies such as Jiaxi, Jinfang Pharmaceutical, and First Pharmaceutical Holdings have also Continuous improvement in the preclinical stage, and strive to accelerate the development of clinical trials of related drugs. Of course, KRAS G12C is not a perfect answer. After all, the problem of resistance always exists, but scientists are also working to improve, such as exploring the combination of KRAS inhibitors and SHP2 inhibitors. How this target will develop in the future, and who will stand out, let us wait and see.







