According to incomplete statistics, as of May 31, 54 financing incidents (excluding IPOs, mergers and acquisitions, equity financing, private placement and strategic investment) occurred in the domestic medical and health field this month, and the financing amount reached 15.599 billion yuan or more (not yet It was revealed that the financing amount was calculated as 0), which was an increase of 26% compared with the previous month. During the period, the medical device field ranked first with a financing amount of 9.183 billion yuan, followed by biomedicine, medical services and digital health. The financing amount was 6.671 billion, 405 million and 490 million (undisclosed financing amount was calculated as 0). ). At the same time, most of the data with the largest financing amount also come from the field of equipment. Compared with the previous month, the field of biomedicine generally lacks major investment projects.
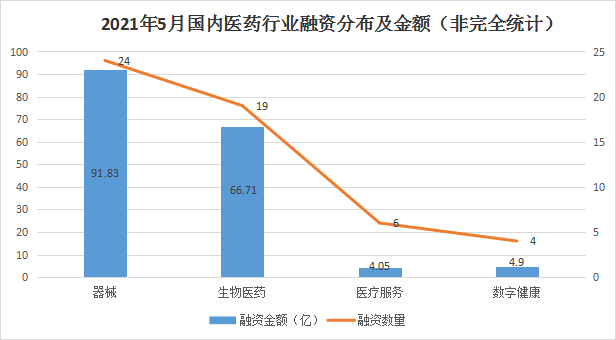
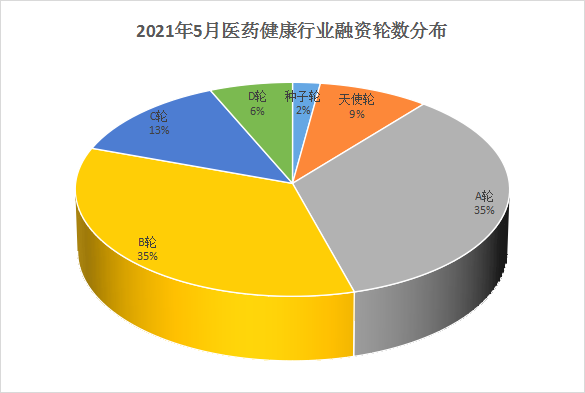
In terms of financing composition, there were 54 domestic financing projects in the medical and health sector in May 2021 (excluding IPOs, mergers and acquisitions, equity financing, private placement and strategic investment), of which the projects were mainly concentrated in the A and B rounds, which together accounted for 70% of total financing events. In addition, it is worth mentioning that this month's pharmaceutical innovation forces are also unwilling to lag behind. The seed round and the angel round together accounted for 11% of the total financing events. They are the only seed round stage company Immune Ark, and the angel round company De Rui Zhi Yao and Su Sheng Biology and West Lake Yungu Intelligent Medicine.
In the 17 financing events in the biomedical field that focused on hot topics in the year, the total amount disclosed was 6.141 billion yuan (excluding IPO, mergers and acquisitions, equity financing, private placement and strategic investment), and more start-up companies focused on details. Field, trying to avoid the frontal competition with large pharmaceutical companies.
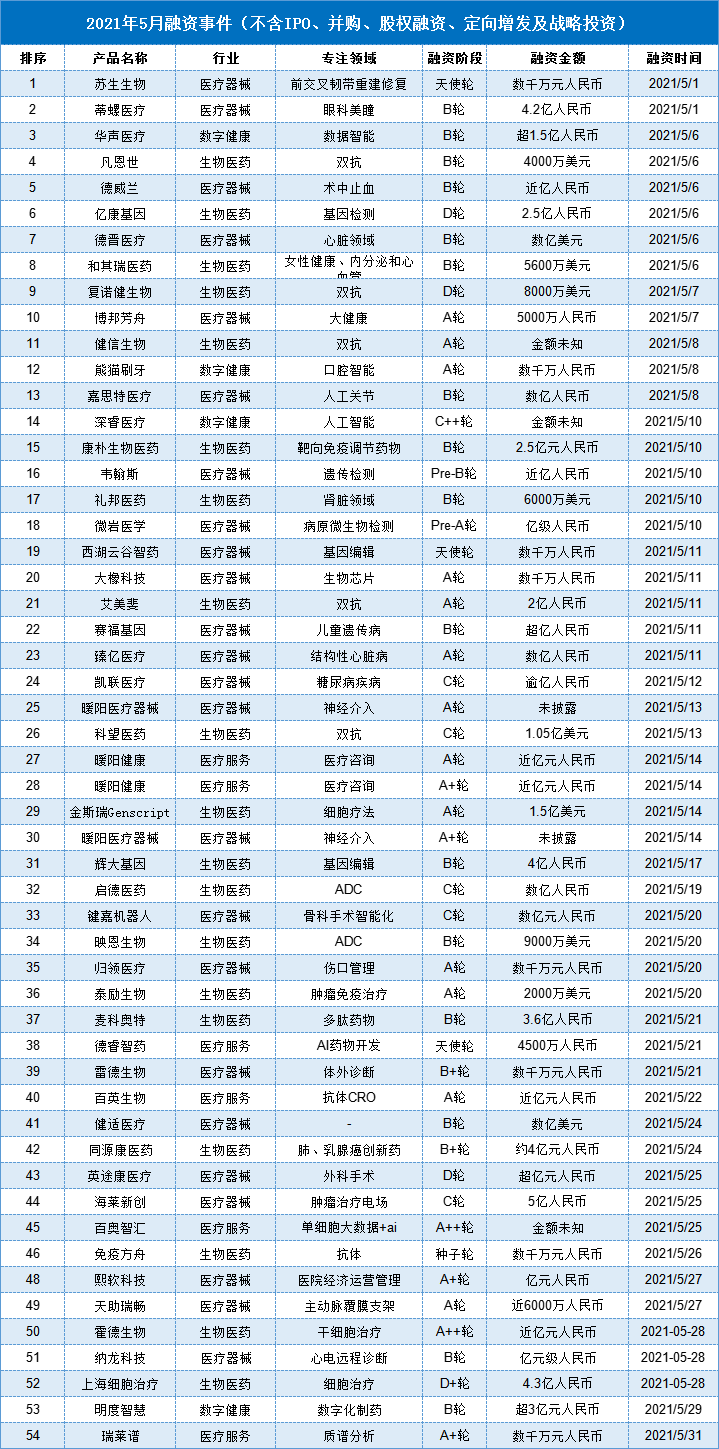
At present, the focus of biomedicine financing is mainly in "tumor immunotherapy", among which double antibodies, ADC and genetic testing are the areas that have received the most attention from innovative companies. This is actually due to the price reduction of PD-1 and the "Technical Guidelines for Similarity Evaluation and Indication Extrapolation of Biosimilars" issued by the official website of the Drug Evaluation Center of the National Medical Products Administration. The crowded situation in the PD-1 field has made the competition between start-ups and established companies become stricter, and indirectly forced a group of Biotech companies to find the next blue ocean market. Nowadays, it seems that double antibodies and ADCs are undoubtedly their best choice.
The concept of "dual resistance" leads this month's financing hotspot
With the gradual deepening of tumor immunity research, more and more drugs have received feedback on the effect of the landing, and the effect of single-agent immune therapy is limited, and the method of immunization plus combination has gradually become the consensus of everyone. Compared with the previously marketed targeted or immune drugs with a single target, the emerging dual-target immune drugs, the so-called "double antibodies", can achieve the effect of inhibiting two targets at the same time with one drug. Not only can the therapeutic effect of immunotherapy reach the "1+1>2" effect, it can also reduce the occurrence of adverse reactions as much as possible. However, compared with monoclonal antibodies, the research and development of double antibodies has not only a high technical threshold, but also a high risk of finished drugs. Even multinational pharmaceutical companies with strong funds are also rich in a lot of bitter history. After Eli Lilly terminated the clinical development of the PD-L1/TIM-3 bi-antibody LY3321367 and one IL-23/CGRP bi-antibody product, on January 19th, its LOXO announced that it would cooperate with Merus to develop three CD3 bi-antibody products drug. In February 2019, the dual antibody product Bintrafusp, jointly developed by GlaxoSmithKline and Merck, also focused on the immune agonist OX40 track. The research and development, cost, and target of double antibodies are the key factors for the successful commercialization of the market. After all, the signal pathways in the human body are extremely complex, and the effect is really difficult to control. However, the strong market demand and potential behind it also make the entire biomedicine Industry entrepreneurs are rushing. According to incomplete statistics, in May of this year alone, seven companies involved double antibodies in the domestic biomedical field financing incident, and each of them has its own unique source advantage.
Kewang Pharmaceutical
Established in 2017, Kewang Pharmaceutical is a biomedical company specializing in tumor immunotherapy. Although it has only been established for more than two years, its outstanding performance has attracted the attention of the industry. Based on the new target discovery platform ElpisourceTM, the tumor immune in vitro drug efficacy screening platform ImmunosineTM, and the bi-antibody design platform AbLegoTM three major medical R&D technology platforms, Kewang Pharmaceutical’s R&D pipeline covers a wide range of tumor immunity and is in the field of natural immunity with great potential. Constructed 12 highly innovative product pipelines. Among them, 30% of the products are drugs based on completely innovative targets, and 70% are differentially designed drugs based on existing targets.
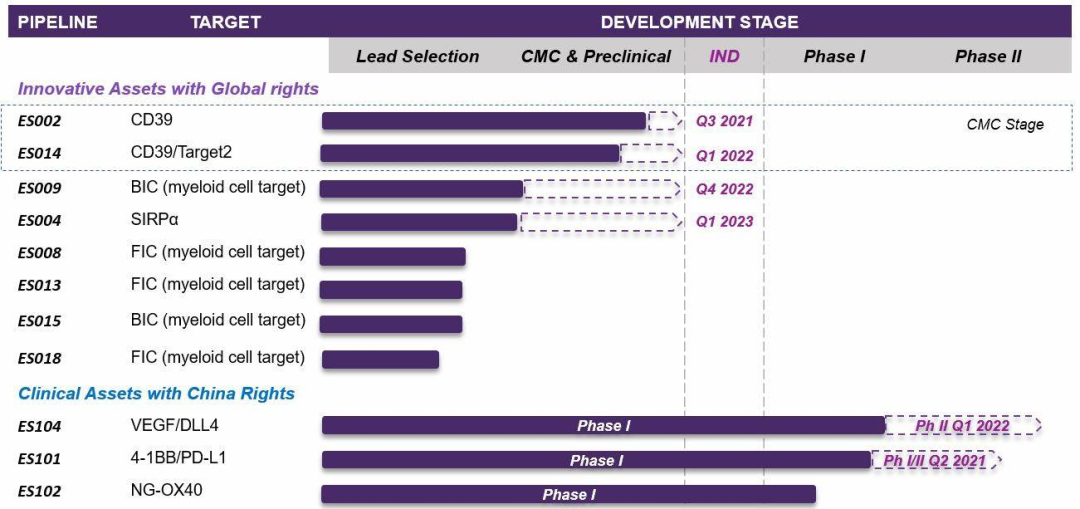
In February 2019, the first product ES101 in Kewang Pharmaceutical's pipeline was approved for clinical trials in China. On June 28, 2019, the first patient administration in China was completed at Shanghai Dongfang Hospital. As the world's first, tetravalent bispecific antibody, it was developed by Inhibrx and has a structure similar to Corning Jereh KN046. It is a quadrivalent bispecific antibody that simultaneously targets PD-L1 and 4-1BB, and has a unique mechanism of PD-L1 binding-dependent activation of 4-1BB.
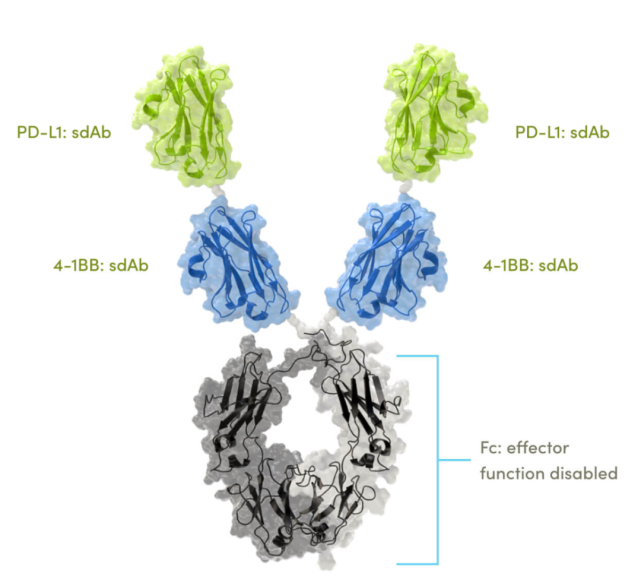
Kewang Pharmaceutical has its rights in Greater China, and Inhibrx retains its rights in the United States and other parts of the world. At present, ES101 has entered the phase I clinical trials in China and the United States. It is also the most promising product of Kewang Pharmaceutical to be listed first. Regarding the future development of Kewang Pharmaceuticals, Vice President Wang Guanhua said, “At present, there are more than a dozen antibodies in Kewang’s product line. For long-term development, we must expand the team’s software and hardware capabilities to control the process, quality, and production. In the future, we hope that two of the product pipelines will enter the process development stage every year, and one or two will apply for IND to enter the clinic, and finally the product will be launched on the market."
Jianxin Bio
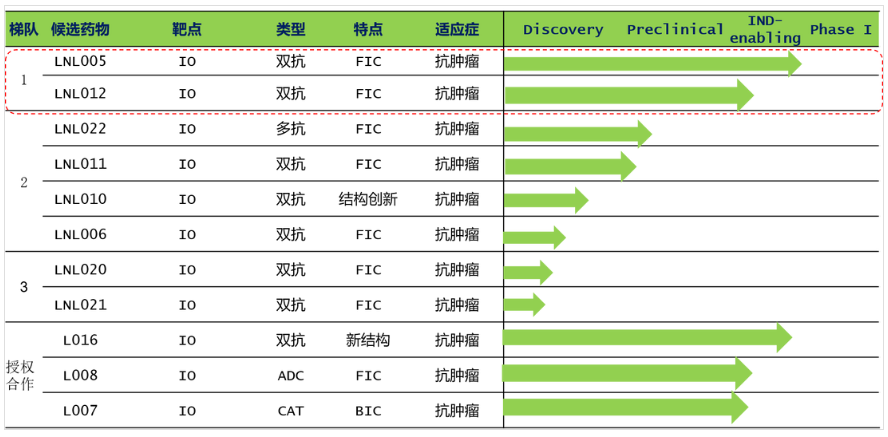
Recently, Shanghai Jianxin Bio-Pharmaceutical Technology Co., Ltd. (referred to as "Jianxin Bio") has received Series A financing. This round of financing was led by Pan Lin Capital, followed by Hongfeng Capital, Qiaojing Capital, and Tuojin Capital. After the financing is completed, Jianxin Bio will accelerate the promotion of the Sino-US IND application and phase I clinical trials of two dual-target products, BIS5 and BIS2. As a high-tech enterprise focusing on the research and development of innovative biomacromolecule drugs. Jianxin Biology focuses on the development of dual-target drugs for tumor immunity. The first core product under development, BIS5, is a dual-antibody product for the treatment of lung cancer. It aims to solve the clinical needs of patients with ineffective and drug-resistant PD-1 treatment, and activate immune cells. It has a differentiated advantage in the group, can effectively relieve immunosuppression, and is expected to become the second-generation product of PD-1. BIS2 is a dual-target immunotherapy product for patients with pancreatic cancer and gastric cancer. The BIS2 molecule is novel in design, has a differentiated mechanism of action, and has shown good efficacy in preclinical animal models.
(Http://lnlbio.com/products2013521697.html)
At the same time, the more than 10 drugs in the Jianxin Biologics R&D pipeline are in different stages of preclinical research and development, forming a well-defined pipeline echelon, but these are mainly developed based on the company's proprietary "S-body" technology platform. In general, Jianxinsheng is considered to be an innovative enterprise that has deployed dual-target tumor immune drugs earlier in China. Its team is relatively forward-looking and unique, and its core products show obvious differentiated design and innovation, with huge potential. Hao Jingjing, Executive Director of Tuojin Capital, said: “In the post-immunotherapy era, dual antibodies have gradually opened up the possibility that they may become the next-generation mainstream products. The core products of Jianxin Biology are deployed here. The current progress of Jianxin Biology’s research and development is orderly. With the advancement, the company's core product leadership and pipeline R&D efficiency are also evident. In the future, Jianxin Bio is expected to become a benchmark company for new-generation antibody drugs.
Venus Biology

On May 6, Phanes Therapeutics (Phanes Therapeutics, hereinafter referred to as Phanes Biopharmaceuticals) announced that the company has completed a US$40 million Series B financing, led by Sequoia Capital China Fund, Deyi Capital, Volcanic Rock Investment, and Kang Jubilee Global Investment Fund and Wenzhou Fund followed suit, and old shareholders Xianfeng Cowin and angel investors made additional investments. This round of financing will comprehensively accelerate the clinical transformation of multiple anti-tumor monoclonal antibodies and double antibody products in the company's R&D pipeline. Since its establishment two years ago, Fintech has focused on the frontier field of dual antibodies, and has applied for 17 patents. Aiming at many technical bottlenecks in the field of dual antibodies, it has established the PACbodyTM natural IgG structurally similar dual antibody platform and the ATACCbodyTM adjustable active dual antibody platform , Developed a product pipeline based on First-In-Class, covering products for various indications such as hematoma, small cell lung cancer, pancreatic cancer, gastric cancer, ovarian cancer and eye diseases. At present, many projects have entered the pre-clinical IND application preparation stage. In the past 2019 and 2020, three license-out external authorization transactions have been concluded, and the company's original innovation capabilities and commercialization prospects have been recognized.
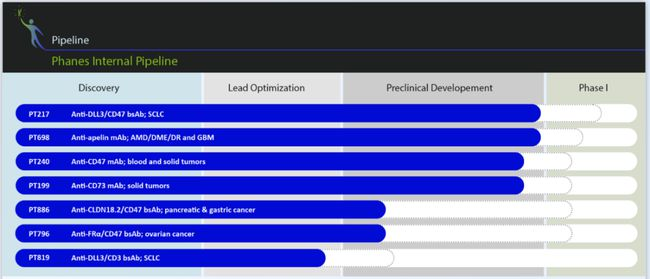
PACbodyTM (pronounced "Packbody") is a dual antibody platform with a similar structure to natural IgG. The Finns Biosystems platform has assembled a number of innovative dual antibody products, further enriching the company's product pipeline for multiple solid tumor indications. At the same time, the ATACCbodyTM (pronounced “Attackbody”) platform is also progressing smoothly. Its feature is that it can adjust the activity of antibodies so that the antibodies are only active in the tumor microenvironment, reducing the risk of cytokine storms, and it is also a belt in the field of immuno-oncology. Here comes a huge transformational product.
Fonogym Biotech and Emifi
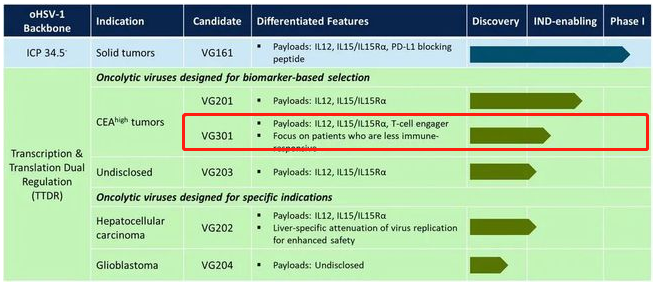
On May 7th, Fonogym Bio, which announced that it had received US$80 million in D1 round of financing, was established in 2015. It is also an innovative company dedicated to the development of a new generation of oncolytic virus products based on tumor immunotherapy. The fastest research progress of VG161 is The new generation of recombinant herpes simplex oncolytic virus is also the world's first oncolytic virus product carrying four immune regulatory factors. VG301 is the world's first HSV-1 oncolytic virus carrying bispecific antibodies.
On May 11, Nanjing Aimefe Biopharmaceutical Technology Co., Ltd. ("Aimefeel"), which announced the completion of 200 million yuan in Series A financing, has led venture capital and Junshi Biotech in its financing team to focus on innovative drugs. Investment professional institutions. Its core management team is composed of former GSK executives, bringing together a group of middle-level technical backbones from well-known pharmaceutical companies such as GSK, Pfizer, and Novartis to form a highly competitive new drug research and development team. In addition to the fast-moving small molecule pipeline in the pipeline, it also has the original frontier layout in the monoclonal antibody and bi-antibody pipelines, forming the second echelon pipeline and obtaining the corresponding original candidate molecules. It is expected to be in the first quarter of 2022. After completing the IND application, it will officially enter the clinical research phase.
summary
Judging from the current research and development of tumor immunotherapy, the next step for monoclonal antibodies is definitely to use combination drugs. Compared with monoclonal antibodies, monoclonal antibodies, chemical drugs, and other target biological drugs, how much higher the efficacy of double antibodies is? Whether the response is much lower than Combo is still unknown. However, a number of investment and financing institutions have also started multi-line layout of early innovative companies, trying to blindly select a dark horse from them, and the ADC field is also one of them. Because they know that when the first "dual antibodies" for solid tumors comes out, they may still have to wait and see, treat calmly, and wait for the results of clinical trials. However, there are more and more "dual antibodies" with more and more stable efficacy. Now in the clinic, real hope is within reach. Perhaps 2021 will be the next breakthrough year for immunotherapy. ADCs, like double antibodies, must be seized.
The concept of "ADC" financing is not diminishing
As hot as double antibodies, another area that has attracted a lot of investment to compete for high-quality resources is undoubtedly the field of antibody-drug conjugate (ADC) research. As a new era that combines highly targeted antibody drugs with powerful chemotherapeutics, precise injection of drugs into tumor cells while avoiding the killing of normal cells by chemotherapeutics, thereby reducing adverse reactions in the treatment process research direction. The first ADC drug Mylotarg was approved for marketing in 2000, but ADC did not usher in an outbreak until 2019. There are currently 12 drugs on the market, as many as 90 ADC drugs are in the clinical stage, and more than 200 are in the clinic Pre-research stage.
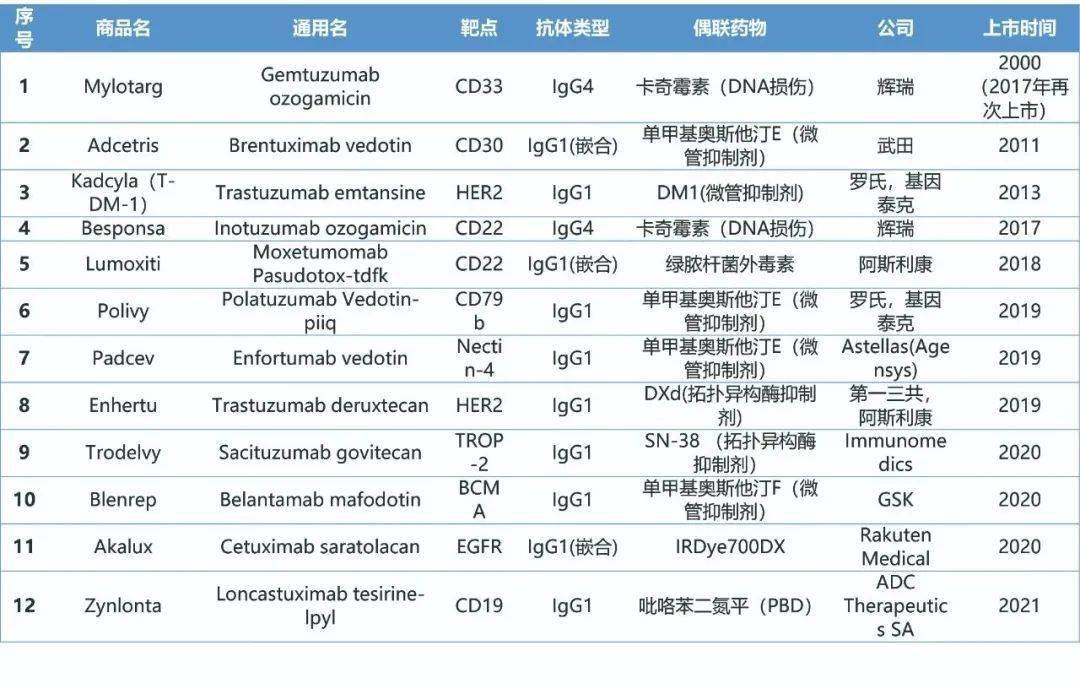
ADC drugs are currently mainly used in the field of tumors. They are similar to other monoclonal antibody drugs in terms of target selection. A large number of specific expression of the target on the surface of tumor cells is basic, and it should also have a certain endocytosis rate and appropriate endocytic transport. way. In addition, the target does not require biological effects, so compared to monoclonal antibodies, ADC drugs have more target choices. Since its discovery, the current ADC research has developed to the third generation. Compared with previous generations of products, the stability and pharmacokinetics are greatly improved, the drug activity is higher, and the toxicity is lower. Related cutting-edge research is not unique to the powerful listed pharmaceutical companies, and the research progress of some small innovative companies is also not to be left behind.
Kai Tak Pharmaceutical
Qi Tak Pharmaceutical, which is led by China Life Health Fund and jointly financed by PICC Capital Equity Investment Company, Sansheng Guojian, and Wuzhong Biomedical Industrial Park, recently completed hundreds of millions of yuan in Series C financing. As a global innovative biopharmaceutical company established in 2013, the core focus is on the development of new bio-conjugated drugs with differentiated innovation platform technology. It has a combination of core technologies authorized by global patents such as enzyme-catalyzed site-specific coupling technology, innovative linker technology, and intelligent continuous coupling (iLDC) platform technology, which effectively solves the current high heterogeneity of ADC drug products, narrow therapeutic window, Commercial production is full of challenges and other issues.
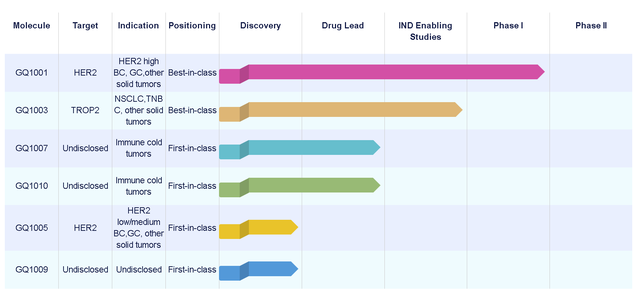
The fastest-growing GQ1001 in the product pipeline of "Qide Pharmaceutical" is an antibody-conjugated drug, which is currently used for the treatment of HER2+ solid tumors in global multi-center clinical trials. It is an ADC produced by the site-specific coupling of toxin DM1 and trastuzumab based on Qi Tak Pharmaceutical’s ligase-catalyzed coupling technology and the patented open-loop linker technology. Using the above technology, the stability and homogeneity of GQ1001 are significantly improved, effectively expanding the treatment window of GQ1001. Qin Gang, Chairman and CEO of Qi Tak Pharmaceutical, also expressed the company’s future prospects, “In the next few years, our iLDC research and production platform will enter an important harvest period, and the team is confident and capable to promote more innovative products in global clinical development. ."
Ying En Biological
On May 20th, another innovative company focused on ADC drug development received US$90 million in Series B financing, led by Eli Lilly Asia Fund (LAV), Coupled Health Fund, Huagai Capital, Newley Capital, Songhe Capital and Yuanhe Holdings and other well-known domestic funds jointly participated in the completion. The funds raised in this round will be mainly used for the company's global advancement of preclinical research, clinical development and product licensing of product pipelines, and the development of innovative molecular type drugs. Focusing on the field of tumors and autoimmune diseases, Yingen Biotech has created a product pipeline with synergistic effects based on the urgent medical needs to be met, including nearly 10 Best-in-class and First-in-class bispecific antibodies and antibodies Conjugated drugs (ADC). In terms of technology platform, In-EnBio has successfully established a next-generation ADC platform with global intellectual property rights. The value of the platform has been verified on a number of drug molecules under development. At the same time, Ingen Bio is further expanding and enriching the novelmodalitydrug platform through independent research and development and external cooperation. Dr. Chen Zhisheng, CEO of WuXi Biologics, said: "The ADC track is currently setting off a wave of R&D and investment. WuXi Biologics has established an ADC drug integration technology platform and service capabilities, and has accumulated rich project experience. We are very much I am pleased to witness the rapid development of Ingen Biotech. In the future, it will continue to empower Ingen Biotech to accelerate the research and development process of innovative ADC drug pipelines, reduce production costs, and accelerate the commercialization process."
summary
In fact, whether innovative companies focus on bi-antis or ADCs, or even both, to a certain extent, they can reflect the focus of today’s pharmaceutical R&D field, and the teams that get the capital market are not just that the products are expected to land. Perhaps more is the sense of glory recognized by the times!













