Heavy! Special procurement of Chinese patent medicines struck, including more than 5 billion varieties
August 18, 2021
Recently, the National Medical Insurance Administration, in reply to the recommendation No. 4126 of the Fourth Session of the Thirteenth National People’s Congress, clearly stated that it will work with relevant departments to improve the quality evaluation standards of Chinese patent medicines and formula granules, adhere to quality first, and be oriented to clinical needs. Starting with high-priced and large-volume varieties, scientifically and steadily promote the reform of centralized procurement of Chinese patent medicines and formula granules. He also said that at present, Qinghai Province, Jinhua, Zhejiang, Puyang, Henan and other places have carried out centralized procurement exploration for some Chinese patent medicines with high demand and high amounts, and positive results have been achieved.
Special collection of Chinese patent medicines started
A week later, the special collection of proprietary Chinese medicines began! Today, the industry circulated a letter about the Hubei Provincial Medical Security Bureau’s invitation to participate in the centralized procurement of Chinese patent medicine provincial cross-regional alliances (hereinafter referred to as the "notification letter"). With the advantages of linking volume purchase with volume and price, Hubei Provincial Medical Security Bureau invited brother provinces (cities) to jointly form a provincial cross-regional alliance to carry out centralized procurement of Chinese patent medicines, which means that the special centralized procurement of Chinese patent medicines has begun. At the same time, it is proposed that provinces that intend to participate in the centralized procurement of Chinese patent medicine provincial cross-regional alliances must reply before 18:00 on August 20, 2021, and specify the alliance's centralized procurement liaison personnel.
Image source: Internet
17 product groups, 74 varieties, over 5 billion large varieties and new crown recommended drugs
According to the notification letter document, a total of 17 product groups (of which Xuesaitong is divided into two dosage forms according to the route of administration) are included in the purchase list, and each product group has one or more drug varieties. According to statistics, a total of 74 products. Among the 74 listed products, oral preparations account for the majority, and there are 6 injections, namely Danshen, Shenmai, Xueshuantong and Xuesaitong, Shuxuening, Shengmai, and Breviscapine.
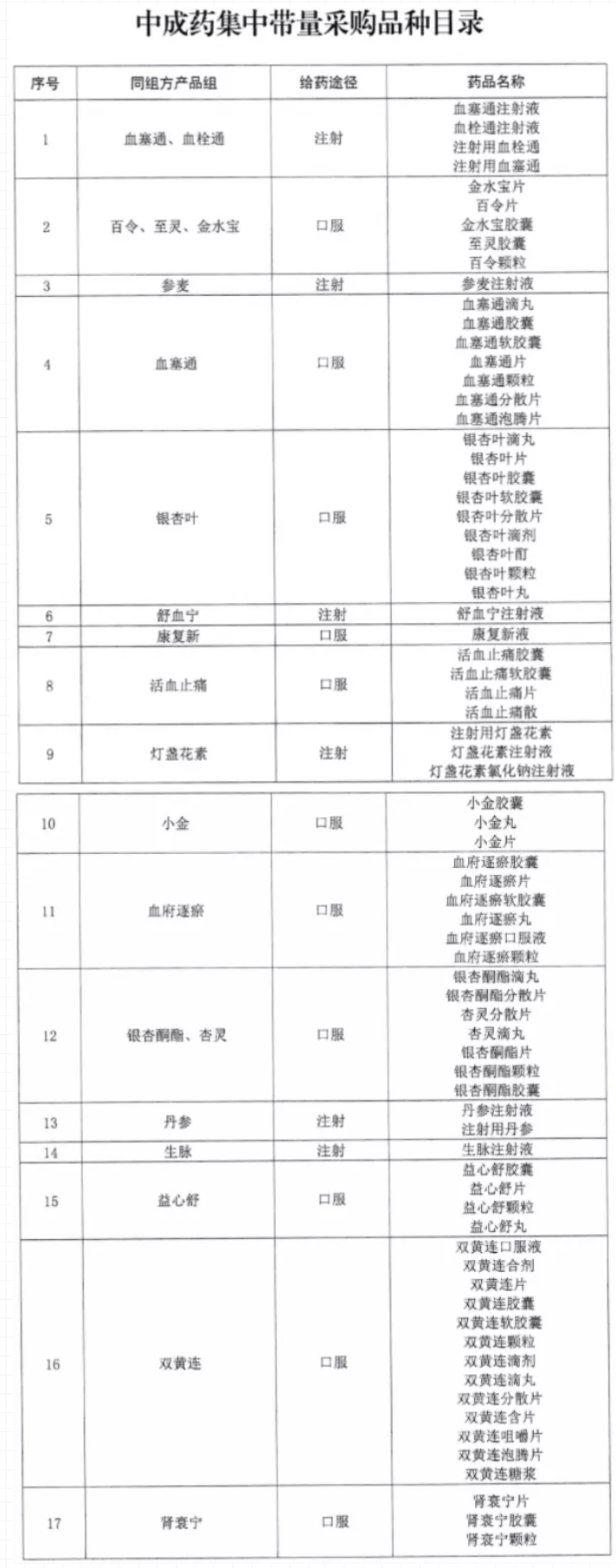
Image source: Internet
It is worth mentioning that Shengmai injection is a Chinese patent medicine recommended by the General Office of the National Health Commission and the State Administration of Traditional Chinese Medicine in the "New Coronavirus Pneumonia Diagnosis and Treatment Program". Internal closure and external exclusion) suffering. Speaking of this, it is surprising that Lianhua Qingwen, who played an important role in the fight against the epidemic, was not seen in the collection list this time. The prescription of Shengmai injection is derived from the classic "Shengmai San", which has the effects of replenishing qi and nourishing yin, restoring pulse and solidifying the offending. It is widely used clinically for symptoms such as qi and yin deficiency, palpitations, shortness of breath, cold limbs, sweating, and desperate pulse. Many guidelines agree to recommend emergency medications for the treatment of myocardial infarction, heart failure, shock and other diseases. According to the data of Yaozhi, there are currently 8 Guochengmai injection companies. In 2020, the sales of domestic sample hospitals will reach 413 million yuan, of which Jiangsu Suzhong Pharmaceutical will account for 32.09%.
Image source: Yaozhi Data
In addition, Xuesaitong injection and Xueshuantong injection have a market size of over 5 billion and large varieties are also on the list. In fact, in recent years, as the country has introduced a variety of measures to promote medical reform and control costs, reduce the proportion of drugs, especially the establishment of auxiliary drugs/key drug monitoring catalogs, most of the injections of Chinese patent medicines are included in the strict control, and they are undoubtedly sold. The amount of growth has an impact. According to Yaozhi data, Xuesaitong/Xueshuantong was included in the list of key monitoring drugs in Henan, Anhui, Shandong and other places. After 2017, the sales data of sample hospitals continued to decline. In 2020, domestic sample hospitals sold Xuesaitong for injection. It is 2.096 billion yuan and Xueshuantong for injection 3.362 billion yuan; of which Xuesaitong for injection Zhenbaodao accounted for 52.58%, and Kunyao accounted for 43.68%. It remains to be seen whether the special collection will be shuffled.
Image source: Yaozhi Data
In fact, the centralized procurement of traditional Chinese medicine is based on quality as the criterion to reshape the market structure of traditional Chinese medicine, which is a supply-side reform of the traditional Chinese medicine industry. Unlike chemical medicines, it is difficult to find a unified standard for the standardization and controllability of Chinese patent medicines, especially the quality standards cannot be the same as chemical medicines to achieve consistent quality and efficacy through consistency evaluation. How to establish standards and systematization is an urgent problem to be solved; An important determinant of the price of Chinese patent medicines is the price of medicinal materials. Unlike chemical medicines, consumables, and even biological medicines, which can be produced on a large scale, they can only be planted, and some may not be able to be planted on a large scale. Large-scale production is used to dilute the cost. Whether the price reduction of proprietary Chinese patent medicines will lead to a shortage of some clinically necessary and cheap Chinese patent medicines, especially how to deal with large varieties, it is worth thinking about. This time, the rules for the centralized procurement of Chinese patent medicines in the Hubei group have not yet been announced. The only way to solve the quality assurance of the centralized procurement of Chinese patent medicines is to wait for the rules to be promulgated. In addition, how should this centralized procurement be carried out? Whether the unit product group wins the bid according to the product group or the variety is also a big question. If the unit product group wins the bid, winning the bid may mean obtaining the full market share... But the specific implementation can only wait for more details to be released!






