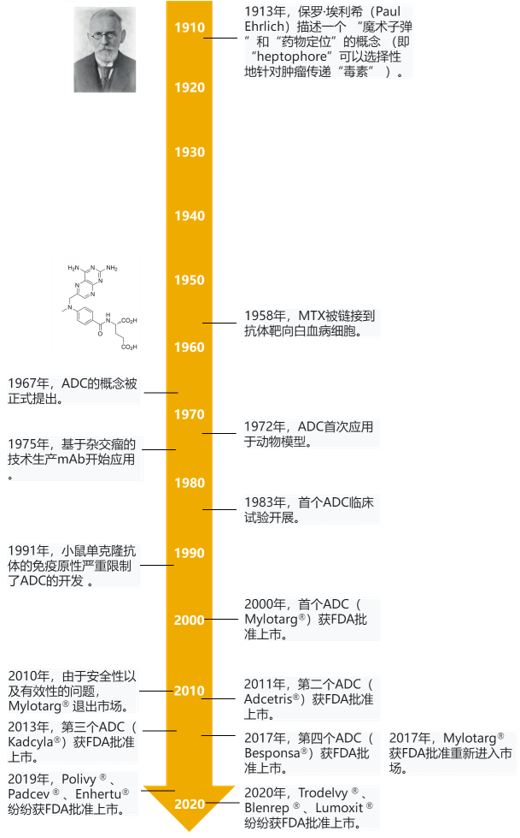
In 2000, the first ADC drug appeared on the market, but it has been tepid. Until 2019, the FDA has approved three ADC drugs (poly ivy, padcev and enhertu), and ADC drugs known as "biological missiles" have entered the outbreak period.
1、 ADC: a "biological missile" for precisely exploding cancer cells
Antibody drug conjugates (ADCs) are new molecules formed by combining traditional small molecule anticancer drugs with recombinant monoclonal antibody (mAb) through linker through chemical reaction (Fig. 1). Generally speaking, these mAbs can specifically recognize tumor specific antigen, so the main purpose of ADC technology is to give some traditional small molecule anticancer drugs active targeting function.

In 2000, FDA approved the first ADC molecule, gemtuzumab ozogamicin, for the treatment of patients with acute myeloid leukemia. Gemtuzumab ozogamicin is a combination of anti-CD33 mAb and calicheamicin, also known as the first generation of ADCs. However, compared with traditional chemotherapy drugs, gemtuzumab ozogamicin did not significantly improve the survival status of patients, and even showed higher lethal toxicity. In 2010, Pfizer withdrew the drug from the market. The reason for the failure may be that ADC is easily affected by the mechanism of drug efflux.
The second generation ADCs are more cautious in the selection of mAbs targeting cancer antigens, thus avoiding the defects of the first generation and having better CMC characteristics. So far, two second-generation ADC molecules have been approved: brentuximab vedotin and trastuzumab emtansine, which target cancer antigen CD30 (also known as tnfrsf8) and human epidermal growth factor receptor 2 (HER2; also known as erbB2), respectively (Fig. 2B, c). These two ADCs are also the only ADCs approved by FDA and EMA.
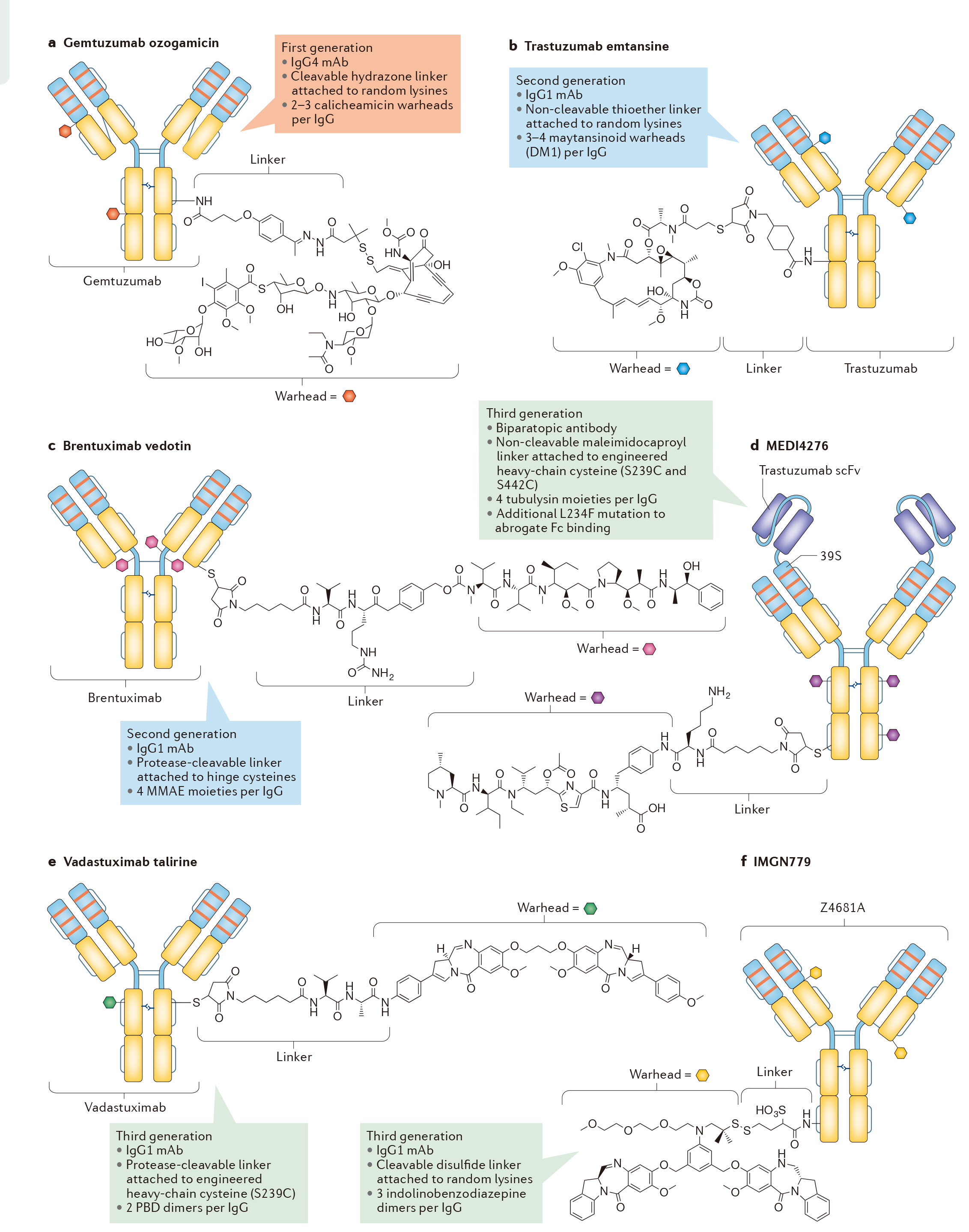
The third generation ADCs further optimized the safety and stability of ADCs molecules, such as medi4276 (Fig. 2D) developed by AstraZeneca / MedImmune, vadastuximab talirine (also known as SGN cd33a; FIG. 2e) developed by little genetics, and imgn779 (Fig. 2f) developed by immunogen. More details of ADC technology development are shown in Figure 3.
2、 New technology creates a market of 100 billion US dollars, and domestic pharmaceutical enterprises speed up the layout of ADC products
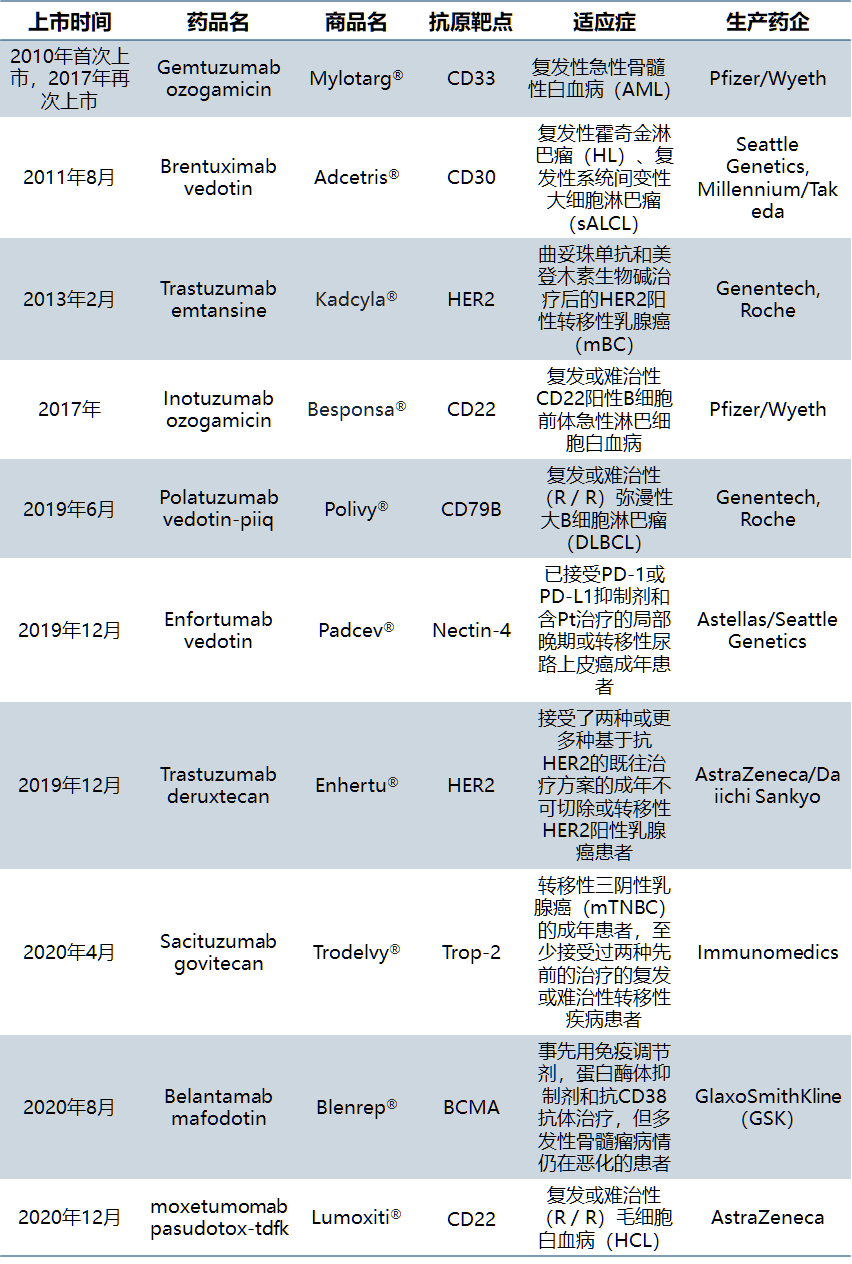
As ADC technology continues to mature, the pace of product launch is accelerated. At present, 10 ADCs products have been approved by FDA and 6 products have been approved in the two years of 2019-2020. Among them, adcetris and kadcyla, which were listed earlier, had sales of US $1.081 billion and US $1.572 billion respectively in 2019, becoming "blockbuster" products. In addition, Polivy, enhertu and trodelvy have the potential to become blockbusters.
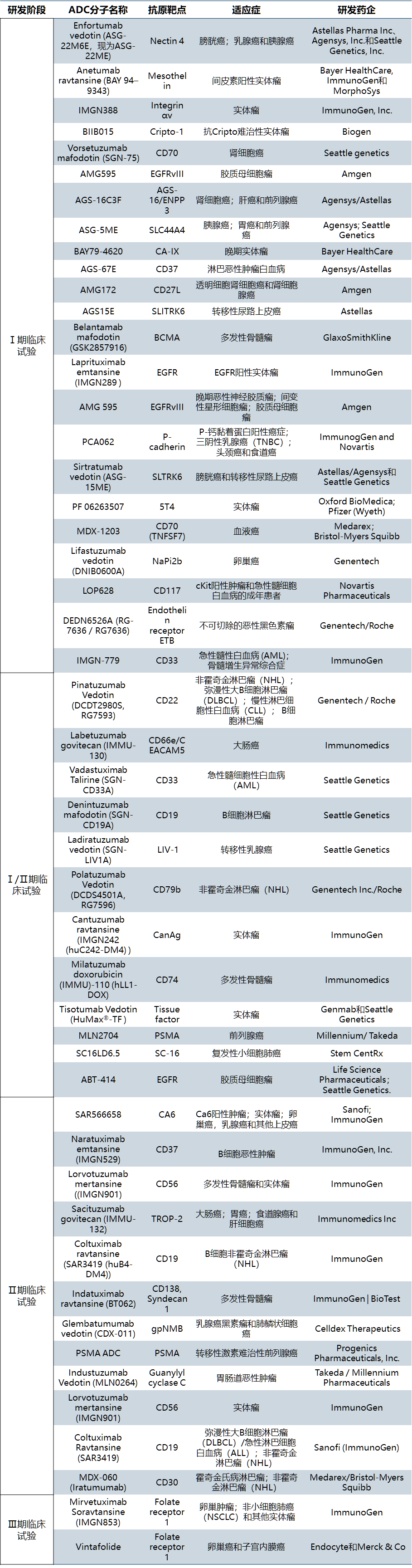
In addition, more than 30 ADCs have entered the clinical development stage (all for cancer indications) abroad, and there are more than 60 ADCs in clinical trials [2]. The first generation, second generation and now third generation ADCs are more uniform, stable and efficient, and have an iterative development process ("from lab to market and back to lab"). See Table 2 for details
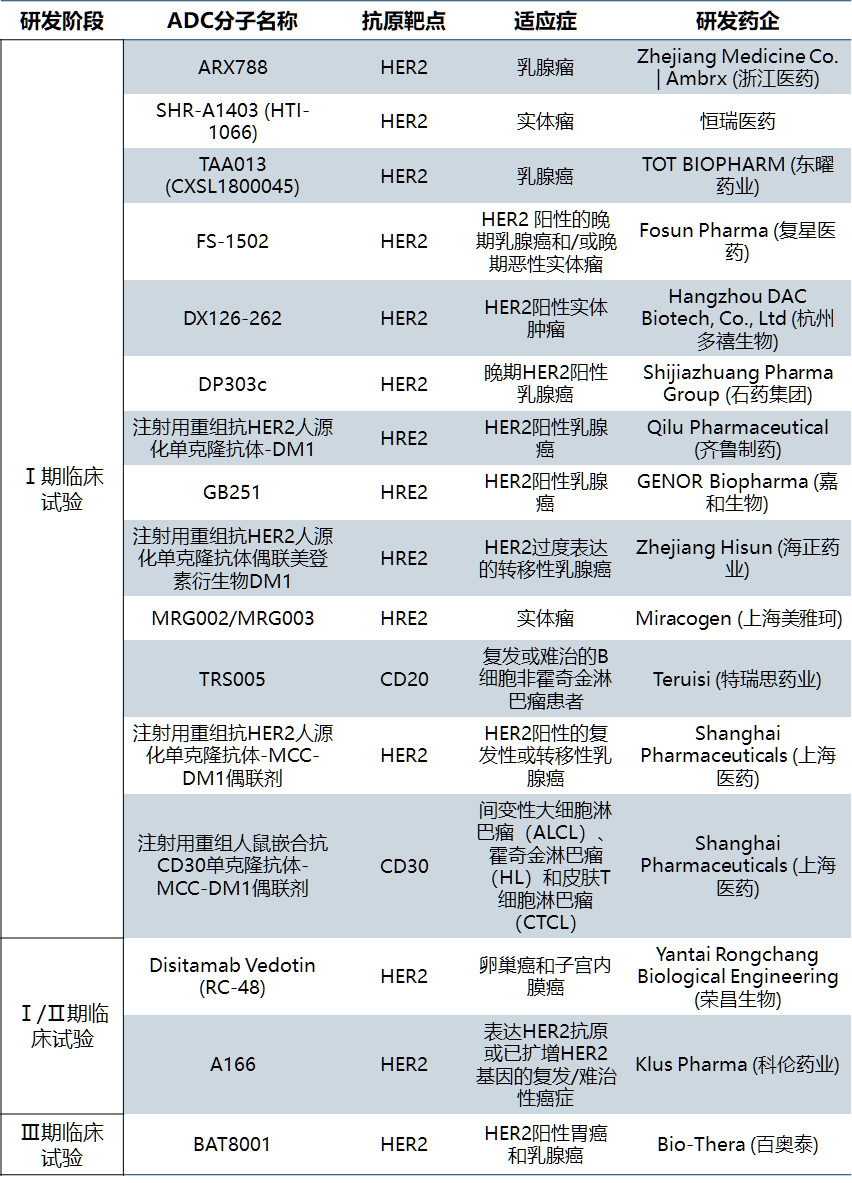
In China, the research on ADCs started late, and the most rapidly developed variety is veditozumab of Rongchang biology, which has been submitted for listing in August 2020. As far as the disclosed projects are concerned, the targets of ADCs drugs in China are relatively concentrated, most of which are the same targets of licensed products or common targets of monoclonal antibody products, and there are few innovative reports on small molecule drug ends, linker molecules and coupling methods. Most of the domestic companies engaged in ADC drug development are transformed from other biological drug businesses such as monoclonal antibody. In recent two years, several companies focusing on ADC technology have emerged, such as Macquarie, lianning biology, nuoling biology, Qide medicine, DUOXI biology, etc. Because there are many preclinical studies and it is difficult to verify, table 3 lists the ADCs drug development enterprises and their projects that have been approved or accepted in China.
3、 Key points and difficulties of ADC drug research and development
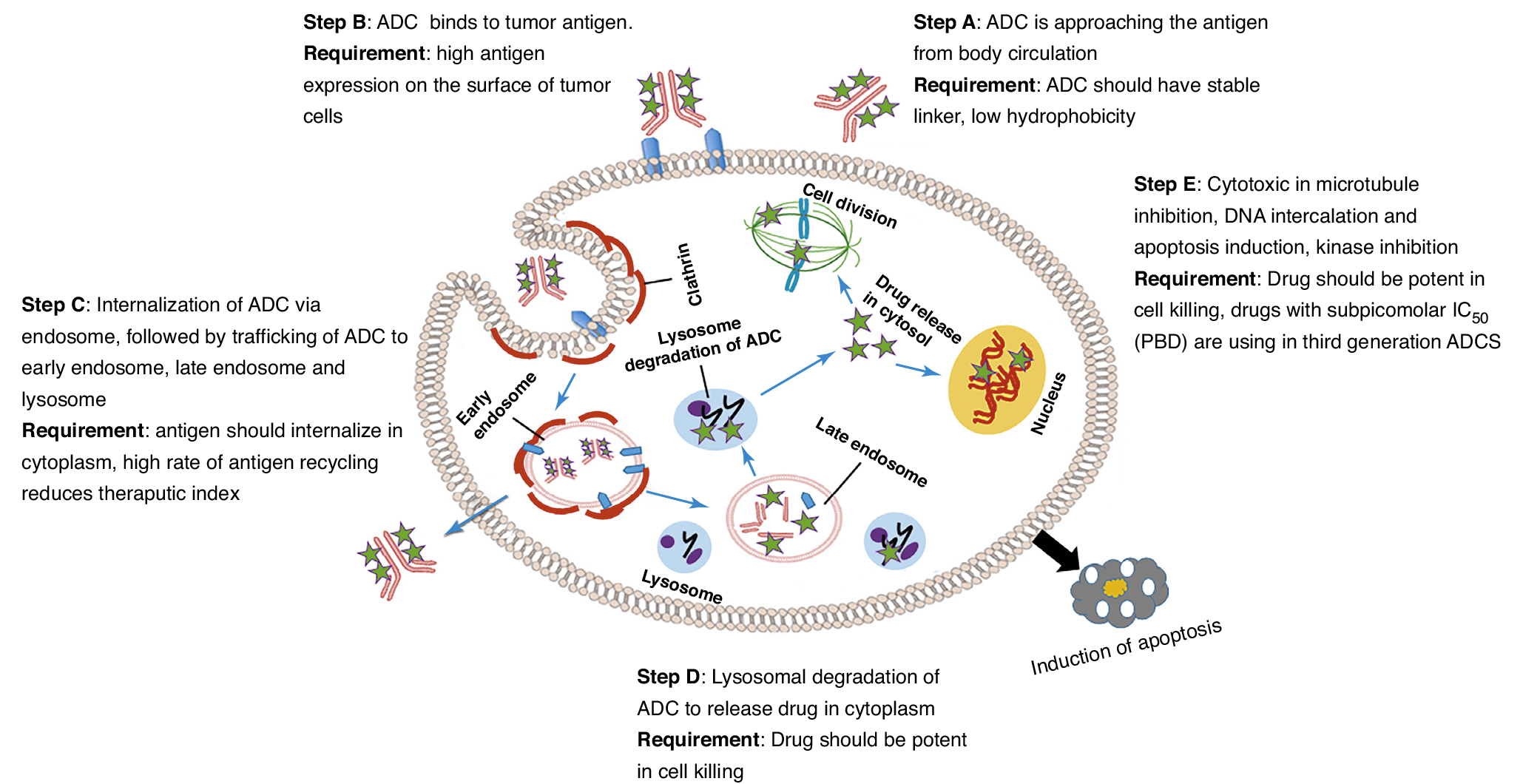
As mentioned above, structurally speaking, ADCs are mainly composed of three parts: traditional small molecule anticancer drugs responsible for killing cancer cells, mAb molecules that can provide targeted effect, and linker. From the mechanism of action, the bright spot of ADCs is targeting. Therefore, the key and difficult point of research and development of ADCs is to achieve drug targeting without affecting the efficacy. So how can ADCs be targeted? How to release the cytotoxic part?
In short, the role of ADCs molecules mainly includes five steps: ① ADCs molecules reach tumor cells through blood circulation. ② The antibody part of ADCs molecule binds to tumor cell specific antigen. ③ Tumor cells phagocytize ADCs and deliver them to lysosomes through intracellular transport. ④ Lysosomes degrade the linker of ADCs and release the cytotoxic part of ADCs. ⑤ The cytotoxic part of ADCs kill tumor cells through various mechanisms. (Figure 4)
① The challenge of ADCs reaching tumor cells
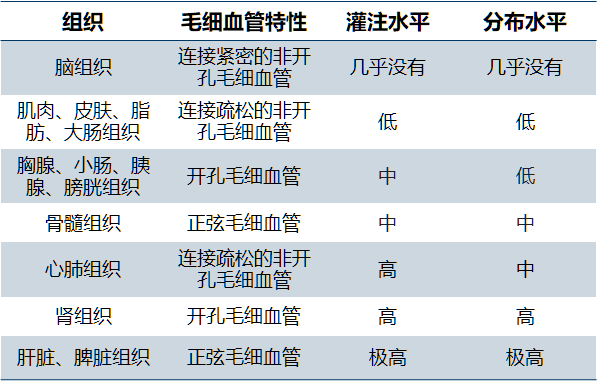
ADCs are usually administered by intravenous infusion. The main risk of subcutaneous administration of ADCs is the stability of linker in skin tissue. Because they are similar in structure to endogenous IgG (generally macromolecules with a molecular weight of about 150 kDa), their distribution is similar to IgG, and they are affected by many of the same physiological processes in vivo. ADCs can reach the target through a variety of mechanisms, such as paracellular, transcytosis and diffusion in interstitial fluid. Its distribution is usually limited to plasma, tissue fluid and lymph nodes. Convective fluid flow promotes the transport of ADCs between these physiological media, such as lymphatic circulation, which can make ADC return from tissue to blood circulation. Due to the different distribution and characteristics of capillaries in different tissues and organs, the distribution of ADCs in different tissues is different (Table 4). For example, because blood convection is completely limited by the blood-brain barrier, the brain is the most inaccessible tissue for ADCs.
② The challenge of ADCs binding to tumor cell specific antigen
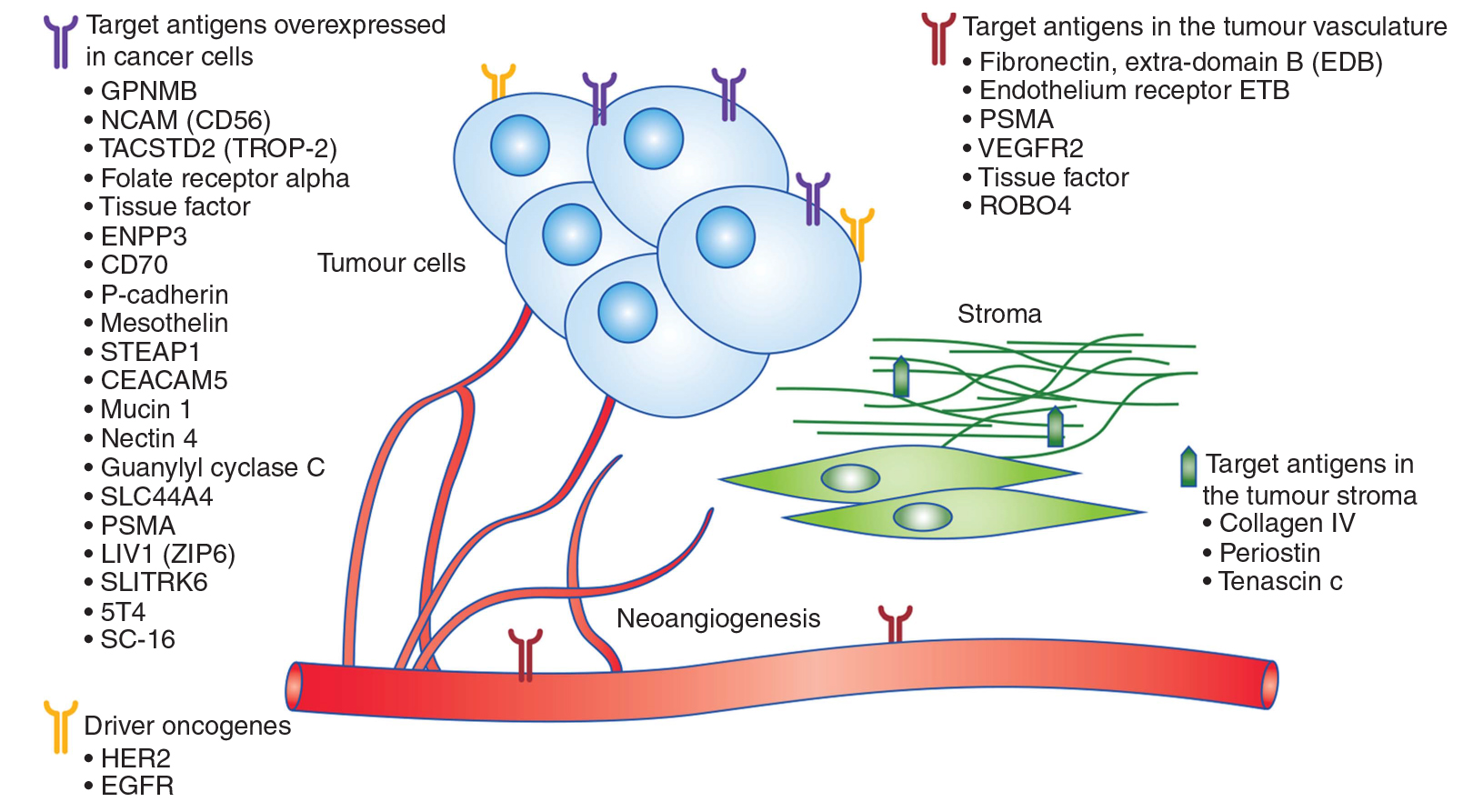
ADCs are designed to bind to the extracellular domain of membrane bound target proteins, and the selection of targeted antigens is the first and most important determinant of successful ADCs. Therefore, based on the difference of target antigen molecules of ADCs in solid tumors, ADCs can be subdivided into ADCs targeting over expressed antigen in cancer cells (such as adcetris), ADCs targeting tumor vascular system antigen (ProstaScint), ADCs targeting tumor stromal antigen, and ADCs targeting antigen driving oncogene regulation (such as kadcyla%) (see Figure 5). In addition to solid tumors, there are also studies on ADCs targeting hematological malignancy antigens. It is suggested that 10000 antigens per cell is the minimum number needed to ensure the selective delivery of lethal cytotoxic drugs to cancer cells.
③ Challenges for tumor cells to phagocytize ADCs
The main challenge of solid tumor therapy is antigen expression, which varies with tumor size, heterogeneity and treatment. In addition to specificity and sufficient expression, the best targeted antigen should also stimulate effective ADC to be phagocytized (internalized) by tumor cells. This internalization efficiency depends on antibody selection, epitope and target type. It has been reported that some targets are often internalized, independent of ligand binding, while others reside permanently on the cell surface [7]. It is generally believed that the anticancer effect of ADC depends on its internalization efficiency by cancer cells. However, recent studies on splice domain fibronectin conjugated meiden lignin alkaloid (SIP f8-ss-dm1) have shown that the internalization level of antigen does not have to reach the therapeutic dose to play the role of ADC [8]. This is because the disulfide linked ADC decreases in the subendothelial extracellular matrix of solid tumor and increases the local concentration of cytotoxic payload near the tumor vascular system, which makes the drug spread to the tumor mass.
④ Challenges of lysosomal degradation of ADCs
The challenge of lysosomal degradation of ADCs is mainly due to the choice of linker. Linker plays a key role in the structure of ADC, because its characteristics greatly affect the therapeutic index, efficacy and pharmacokinetics of ADC. The stable linker in ADC can maintain the concentration of ADCs molecules in the blood circulation, and will not release cytotoxic drugs before reaching the target, so as to minimize the Miss effect. However, once ADC is engulfed in tumor cells, linker should be unstable enough to rapidly release cytotoxic drugs. Another key consideration is how many drug molecules should be loaded into ADCs, which is called drug antibody ratio (DAR). Therefore, it is necessary to optimize Dar, because too few drug molecules will lead to reduced efficacy, while too many molecules will change pharmacokinetics and further increase instability and toxicity. The DAR value of most common ADCs is close to 4.
⑤ The challenge of immunogenicity
The important characteristics of ABS in ADC include antigen affinity, target specificity, good retention, minimal immunogenicity, low cross reactivity and circulation along plasma. Generally, the binding affinity of AB component of ADC is in the range of 0.1 – 1 nm. Mouse antibodies are used in the first generation of ADCs, which cause severe immunogenicity by producing human anti mouse antibodies in patients. However, bioengineering studies have found chimeric, humanized and completely human antibodies. Today, most of the mAbs used by humans are human derived or humanized. In addition to the complement determining region (CDR) of mouse origin, humanized antibodies mainly come from human origin. In the humanized antibody, mouse CDR was transplanted or resurfaced to the surface and constant region of human FV.
4、 Summary
With the continuous iteration and maturity of ADC technology, ADC drugs have achieved better and better results in treatment. At present, a number of ADC drugs on the market show the potential of a blockbuster, and many ADC drugs are in the late clinical stage. Some research institutions predict that the whole ADC drug market is expected to exceed 50 billion US dollars.
In March 2020, Roche's kadcyla was approved to be listed in China. At the same time, the first domestic ADC drug, Rongchang bio's veditozumab, has been accepted by CDE, marking the first year of the commercialization of ADC drugs in China. With the participation of more and more innovative pharmaceutical companies, this field is expected to enter the golden stage of development.
reference material:
[1] Beck, Alain, et al. "Strategies and challenges for the next generation of antibody–drug conjugates." Nature reviews Drug discovery 16.5 (2017): 315-337.
[2] Mullard, A. Maturing antibody–drug conjugate pipeline hits 30. Nat. Rev. Drug Discov. 12, 329–332 (2013).
[3] Development of antibody drug conjugate (ADC)
[4] Sau, Samaresh, et al. "Advances in antibody–drug conjugates: a new era of targeted cancer therapy." Drug discovery today 22.10 (2017): 1547-1556.
[5] Malik, Paul, et al. "Pharmacokinetic considerations for antibody-drug conjugates against cancer." Pharmaceutical research 34.12 (2017): 2579-2595.
[6] Diamantis, Nikolaos, and Udai Banerji. "Antibody-drug conjugates—an emerging class of cancer treatment." British journal of cancer 114.4 (2016): 362-367.
[7] novel anti-cancer chemotherapeutics. Biosci. Rep. 35, e00225
[8] Perrino, E. et al. (2014) Curative properties of noninternalizing antibody-drug conjugates based on maytansinoids. Cancer Res. 74, 2569–2578











