54 varieties have been reviewed! 15 varieties are the first to have reviewed, Nanjing Zhengda has the most sunny days, Yichang Renfu, Qilu...
September 03, 2021
According to the consistency evaluation analysis system of Yaozhi Data Enterprise Edition, 68 new acceptance numbers for consistency evaluation were added in August 2021; 77 approvals (including 9 deemed approved approvals) were over-evaluated. (Attached to the end of the article is a detailed form of consistency evaluation of the application and over-evaluation in August)
Figure 1 Trend of filing/approval from January 2021 to August 2021
Data source: Yaozhi data, Yaozhi consultation
Review details
In August, a total of 77 acceptance numbers passed/deemed passed the consistency evaluation, involving 54 varieties of 49 companies, of which 15 varieties were the first to pass the evaluation. In terms of over-evaluated companies, Nanjing Chia Tai Tianqing Pharmaceutical Co., Ltd. ranked first in August, with 5 varieties passing the consistency evaluation. Followed by Tianjin Jinyao Pharmaceutical Co., Ltd., 4 varieties passed the consistency evaluation. Both Yichang Renfu Pharmaceutical Co., Ltd. and Qilu Pharmaceutical (Hainan) Co., Ltd. have been evaluated for 3 varieties, and are tied for third place. Nanjing Chia Tai Tianqing Pharmaceutical Co., Ltd. was established in 2001, invested and established by four shareholders of Thailand Chia Tai, Chia Tai Tianqing, Jiangsu Agricultural Reclamation, and Nanjing Jinkang. Now it has developed into a trinity of "drug research and development, production, and sales". The products cover the mind and brain. One of the top 100 Chinese chemical companies with comprehensive strength in the treatment fields of blood vessels, tumors, perioperative surgery, digestion, and urinary surgery! There are more than 100 R&D pipelines, hundreds of various patents, and a number of major scientific and technological projects at the national, provincial and municipal levels. The company has 85 approval documents in China's listed drug database, involving 56 varieties. The company has currently declared/deemedly declared 45 varieties, and 24 varieties including irbesartan hydrochlorothiazide tablets, gemcitabine hydrochloride for injection, and apixaban tablets have been evaluated (including deemed approved). Tianjin Jinyao Pharmaceutical Co., Ltd. (hereinafter referred to as Jinyao Pharmaceutical Co., Ltd.) is a holding subsidiary of Tianjin Tianyao Pharmaceutical Co., Ltd., a subsidiary of Tianjin Jinyao Group Co., Ltd., and a key preparation manufacturer and a backbone enterprise in strategic transformation of the group. . At present, Jinyao Pharmaceutical has 16 dosage forms, including small-volume injections, ointments, creams, coatings, gels, films, suppositories, hard capsules, pills, freeze-dried powder injections, and more than 200 product specifications. Approval number of the drug. The company has already declared/deemedly declared 18 varieties, of which 8 varieties such as methylprednisolone sodium succinate for injection and celecoxib capsules have been evaluated (including deemed passed).
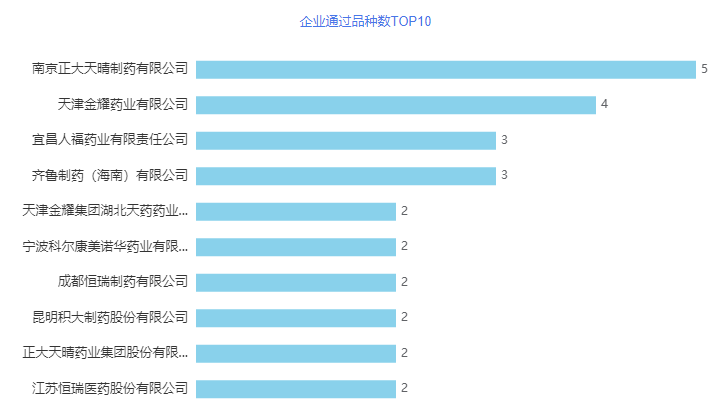
Figure 2 The TOP10 products passed by enterprises in August 2021
Data source: Yaozhi data, Yaozhi consultation
In terms of varieties, the most intensely competitive variety in August was Captopril tablets, and three companies scrambled to comment. In addition, Clindamycin Phosphate Injection and other varieties have been reviewed by 2 companies. Up to now, a total of 40 companies in Captopril have applied for consistency evaluation, of which 18 companies have passed the evaluation. Captopril tablets are indicated for: hypertension, heart failure.
Figure 3 TOP 10 companies passing the variety of products in August 2021
Data source: Yaozhi data, Yaozhi consultation
48 varieties are declared, injections exceed 60%
In August 2021, CDE added 68 acceptance numbers for consistency evaluation, involving 48 varieties of 39 companies, of which injections accounted for more than 60% again.
Figure 4 Details of dosage forms declared in August 2021
Data source: Yaozhi data, Yaozhi consultation
In terms of the declared varieties, in August 2021, three acceptance numbers for glycine injection, piperacillin sodium and tazobactam sodium for injection, and cefmetazole sodium for injection were undertaken. See the picture below for details
Figure 5 TOP10 Number of Variety Application Acceptance Numbers in August 2021
Data source: Yaozhi data, Yaozhi consultation
Aminocaproic acid injection is suitable for the prevention and treatment of various bleeding caused by hyperfibrinolysis. (1) Traumatic or surgical bleeding of organs rich in plasminogen activator such as prostate, urethra, lung, liver, pancreas, brain, uterus, adrenal gland, thyroid, etc., tissue plasminogen activator (t-PA), chain Bleeding caused by overdose of kinase or urokinase. (2) Late stage of diffuse intravascular coagulation (DIC) to prevent secondary hyperfibrinolysis. (3) It can be used as an adjuvant treatment for bleeding or menorrhagia after tooth extraction or oral surgery in patients with hemophilia. (4) It can be used for the symptomatic treatment of various bleeding such as upper gastrointestinal bleeding, hemoptysis, primary thrombocytopenic purpura and leukemia. It has a significant effect on general chronic bleeding; it has a poor effect on bleeding caused by abnormal coagulation function; (5) Local application: 0.5% solution is used to flush the bladder for postoperative bladder bleeding; 10% solution can be used to rinse the mouth and a cotton ball dipped in medicine to fill the wound after teeth extraction; 5% to 10% solution gauze can also be used to apply the wound after soaking. At present, 4 companies have applied for conformity evaluation of this product. It is worth looking forward to who is the first to pass the evaluation. Piperacillin sodium and tazobactam sodium for injection is a compound drug composed of penicillin antibacterial drug piperacillin and β-lactamase inhibitor tazobactam. It is suitable for the treatment of community-acquired pneumonia and hospital-acquired pneumonia Moderate to severe infections caused by susceptible isolates of designated bacteria in diseases such as urinary tract infections. In order to reduce the production of drug-resistant bacteria and maintain the effectiveness of this product and other antibacterial drugs, this product should only be used to treat or prevent infections suspected of being caused by bacteria with proven or conclusive evidence. There are 74 approvals for this product in China, involving 23 companies. At present, 7 companies have applied for consistency evaluation, and Qilu and North China Pharmaceutical have passed the evaluation. Cefmetazole sodium for injection is suitable for the treatment of Staphylococcus aureus, Escherichia coli, Pneumoniae The following infections caused by the genus Coccus and Provobacterium (except two-way Prevotella): sepsis; acute bronchitis, pneumonia, lung abscess, empyema, secondary infections of chronic respiratory diseases; cystitis, pyelonephritis; Peritonitis; cholecystitis, cholangitis; bartholinitis, intrauterine infection, uterine adnexitis, parauterine tissue inflammation; cellulitis around the jaw, jaw inflammation. At present, China has 70 approvals for this product, involving 28 companies. At present, 8 companies have applied for consistency evaluation, of which only Chengdu Beite has passed the evaluation. From a corporate perspective, in August Sichuan Baojiantang Pharmaceutical Co., Ltd. and Chengdu Better Pharmaceutical Co., Ltd. had three acceptance numbers for joint declaration consistency evaluation accepted, ranking first on the list. Many companies have two acceptance numbers that have been accepted for consistency evaluation. See the figure below for details.

Figure 6 TOP10 number of enterprise application acceptance numbers in August 2021
Data source: Yaozhi data, Yaozhi consultation
Sichuan Baojiantang Pharmaceutical Co., Ltd. was formed by the acquisition of Haike Pharmaceutical Branch of Sichuan Shenghe Pharmaceutical Co., Ltd. with the investment of Chengdu Better Pharmaceutical Group. The company has four production workshops, including traditional Chinese medicine extraction workshop, solid preparation workshop, liquid preparation workshop, and bulk medicine workshop, with a total of eight production lines. Sichuan Baojiantang Pharmaceutical Co., Ltd. has currently declared/deemed to declare 2 varieties, and has jointly declared 2 varieties with Chengdu Better Pharmaceutical Co., Ltd., and none of them has been evaluated. Chengdu Better Pharmaceutical Co., Ltd. is a high-tech enterprise specializing in the research and development, production and sales of high-end generic drugs, innovative drugs, Chinese patent medicines and APIs. After years of innovation and development, it has gradually developed into an innovative pharmaceutical company with advanced R&D concepts, strong R&D strength, complete product pipelines, excellent production quality and a sound marketing network. The company has 308 approval documents in China's listed drug database, involving 182 varieties. The company has currently declared/deemedly declared 67 varieties, and 32 varieties including propofol tenofovir fumarate tablets, levetiracetam injection concentrated solution, and ampicillin sodium for injection have been evaluated (including video Same through).
Attached Table 1: Details of the consistency evaluation passed (including deemed passed) in August 2021
Attached Table 2: Details of the consistency evaluation of the declaration in August 2021
Data as of September 1, 2021
Data source: Yaozhi Data Generic Drug Consistency Evaluation and Analysis System










